Consider the acid catalyzed hydration of 3 methyl 1 butene – Consider the acid-catalyzed hydration of 3-methyl-1-butene, a captivating chemical transformation that unveils the intricate interplay between acid catalysts and organic substrates. This reaction holds immense significance in organic chemistry, paving the way for the synthesis of valuable compounds and offering insights into fundamental reaction mechanisms.
Embark on an enthralling journey as we delve into the nuances of this remarkable process, exploring its applications, intricacies, and practical considerations.
The acid-catalyzed hydration of 3-methyl-1-butene is a prototypical example of electrophilic addition, a fundamental reaction type in organic chemistry. This reaction involves the addition of water to the double bond of 3-methyl-1-butene, facilitated by an acid catalyst. The regioselectivity and stereochemistry of this transformation are governed by a complex interplay of electronic and steric effects, providing a rich platform for mechanistic investigations.
Acid-Catalyzed Hydration: Consider The Acid Catalyzed Hydration Of 3 Methyl 1 Butene
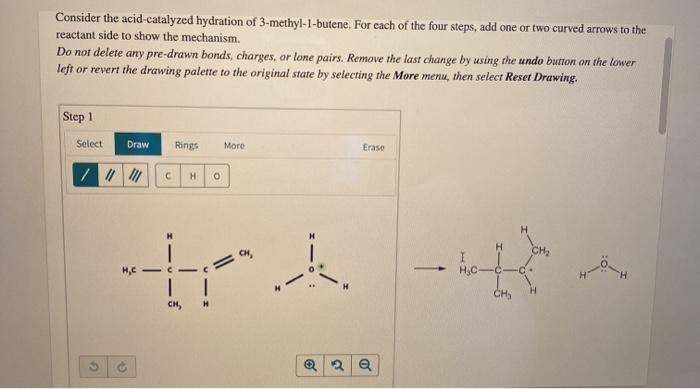
Acid-catalyzed hydration is a chemical reaction in which an alkene (a hydrocarbon with a carbon-carbon double bond) reacts with water to form an alcohol. The reaction is catalyzed by an acid, which donates a proton to the alkene, making it more reactive towards water.
Mechanism of Acid-Catalyzed Hydration, Consider the acid catalyzed hydration of 3 methyl 1 butene
The mechanism of acid-catalyzed hydration involves the following steps:
- The acid donates a proton to the alkene, forming a carbocation.
- Water attacks the carbocation, forming an oxonium ion.
- The oxonium ion rearranges to form an alcohol.
Role of the Acid Catalyst
The acid catalyst plays a crucial role in the reaction by:
- Donating a proton to the alkene, which makes it more reactive towards water.
- Stabilizing the carbocation intermediate.
Examples of Acid-Catalyzed Hydration Reactions
Some examples of acid-catalyzed hydration reactions include:
- The hydration of ethene to form ethanol
- The hydration of propene to form isopropanol
- The hydration of butene to form butanol
Hydration of 3-Methyl-1-Butene
The hydration of 3-methyl-1-butene is a specific example of an acid-catalyzed hydration reaction. In this reaction, 3-methyl-1-butene reacts with water in the presence of an acid catalyst to form two products: 3-methyl-2-butanol and 2-methyl-2-butanol.
Products of the Reaction
The two products of the reaction are:
- 3-methyl-2-butanol (major product)
- 2-methyl-2-butanol (minor product)
The formation of these products can be explained by Markovnikov’s rule, which states that in the addition of an unsymmetrical alkene to a polar molecule, the proton from the polar molecule adds to the carbon atom of the double bond that has the most hydrogen atoms.
Stereochemistry of the Reaction
The hydration of 3-methyl-1-butene is a stereoselective reaction, meaning that it produces one enantiomer of the product in excess over the other. The major product, 3-methyl-2-butanol, is formed with the (R) configuration.
Factors Affecting the Reaction
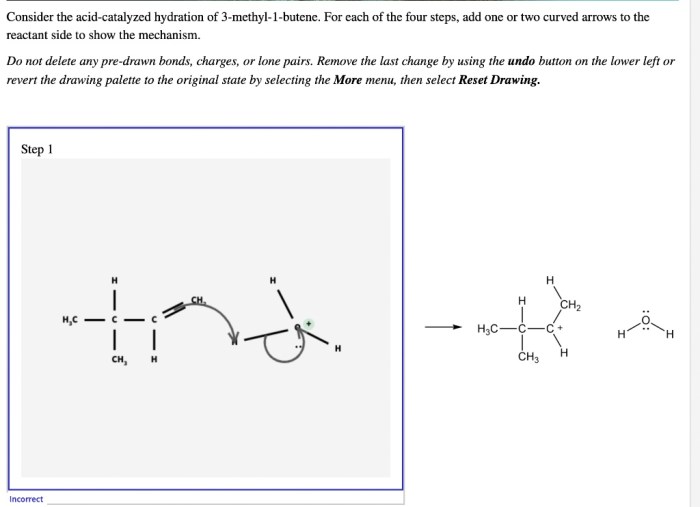
Several factors can affect the rate and regioselectivity of the acid-catalyzed hydration of 3-methyl-1-butene, including:
- Temperature
- Solvent
- Substituents on the alkene
Temperature
The rate of the reaction increases with increasing temperature. This is because the higher temperature provides more energy to the reactants, which allows them to overcome the activation energy barrier and react more quickly.
Solvent
The solvent can also affect the rate and regioselectivity of the reaction. Polar solvents, such as water, favor the formation of the Markovnikov product, while nonpolar solvents, such as hexane, favor the formation of the anti-Markovnikov product.
Substituents on the Alkene
Substituents on the alkene can also affect the rate and regioselectivity of the reaction. Electron-withdrawing substituents, such as halogens, decrease the rate of the reaction and favor the formation of the Markovnikov product. Electron-donating substituents, such as alkyl groups, increase the rate of the reaction and favor the formation of the anti-Markovnikov product.
Applications of Acid-Catalyzed Hydration
Acid-catalyzed hydration reactions have a wide range of industrial applications, including:
- The production of alcohols
- The production of ethers
- The production of aldehydes
- The production of ketones
Limitations and Challenges
Despite their wide range of applications, acid-catalyzed hydration reactions also have some limitations and challenges, including:
- The reactions can be slow, especially in the absence of a catalyst.
- The reactions can be sensitive to the reaction conditions, such as temperature and solvent.
- The reactions can produce unwanted side products.
Safety Considerations
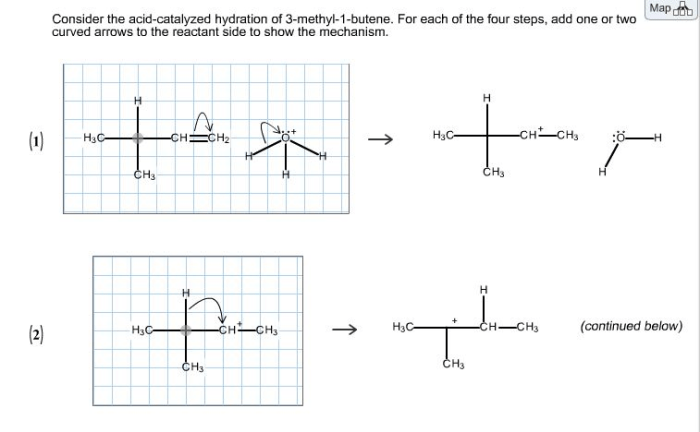
Acid-catalyzed hydration reactions can be hazardous, and it is important to take the following safety precautions when performing these reactions:
- Wear appropriate personal protective equipment, such as gloves, goggles, and a lab coat.
- Handle acids with care and avoid contact with skin and eyes.
- Use a fume hood when working with volatile chemicals.
- Dispose of chemicals properly according to local regulations.
Questions and Answers
What is the role of the acid catalyst in the hydration of 3-methyl-1-butene?
The acid catalyst activates the water molecule, making it a more effective electrophile. It protonates the oxygen atom of water, creating a more electrophilic species that can attack the double bond of 3-methyl-1-butene.
What are the products of the hydration of 3-methyl-1-butene?
The products of the hydration of 3-methyl-1-butene are two regioisomers: 3-methyl-2-butanol and 2-methyl-2-butanol. The major product is 3-methyl-2-butanol, which follows Markovnikov’s rule.
What factors affect the regioselectivity of the hydration of 3-methyl-1-butene?
The regioselectivity of the hydration of 3-methyl-1-butene is affected by several factors, including the nature of the acid catalyst, the solvent, and the temperature. The use of a stronger acid catalyst and a more polar solvent favors the formation of 3-methyl-2-butanol.